New Insights on Activity-Dependent Gene Expression and the Developing Brain
By Jonathan Schneiderman
When neurons are active, the expression of their genes impacts the development of the entire cluster in which they operate. Analyzing gene expression patterns in isolated neurons is relatively straightforward; however, studying them in the context of the brain, especially in humans, is complicated by the presence of many different neuronal subtypes that exhibit unique expression profiles.
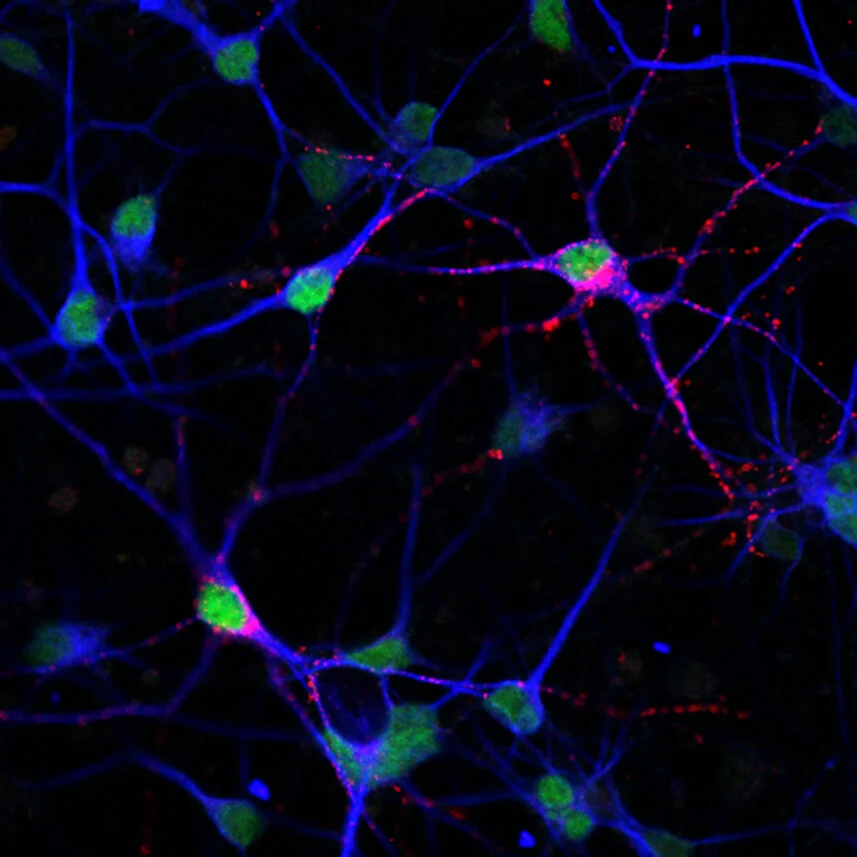
Human stem cell-derived GABAergic neurons that have been depolarized (as a proxy for studying neuronal activity). The green staining identifies activity-dependent phosphorylated form of the transcription factor CREB (pCREB), and the red staining identifies somatostatin (SST), an inhibitory neurotransmitter whose expression is also induced by depolarization. Neuronal cell bodies and dendrites are shown in blue, with staining for the cytoskeletal protein Microtubule Associated Protein 2 (MAP2).
Circuits of neurons that utilize the neurotransmitter GABA are known as GABAergic neurons (GNs) and had previously been implicated in neurological disorders. Gabriella Boulting from the Greenberg lab and colleagues set out to study the activity-dependent expression profile of human GNs using used stem-cell derived neurons, which they profiled before and after stimulation (Nature Neuroscience, 2021). Through these experiments, the team was able to identify disease-related genes that were not previously known to be regulated by neuronal activity. Among the identified genes, the team found several associated with neurological conditions, such as autism spectrum disorder (ASD) and schizophrenia (SCZ).
Using single-cell techniques, the researchers verified their results and found that these immature human GNs, which are similar to fetal spiny projection neurons, induce the expression of a disproportionately large number of ASD-associated genes. They also discovered that inducible promoters—but not inducible enhancers—in these cells have a large enrichment in ASD heritability, while no such association was found for SCZ. The activation of these promoters provides important insights into the unique vulnerability of the developing neurons of the striatum to ASD-associated genetic variation. It can also help explain how disruption of neuronal activity-dependent signaling could contribute to ASD risk.
In a separate, yet related, line of investigation, Hume Stroud (also from the Greenberg lab) and colleagues identified a postnatal switch in transcriptional regulation in the developing mouse brain by purifying neuronal subpopulations at different developmental stages (Neuron, 2020). They found that the transcriptional transition, which is thought to be impaired in neurological disorders like ASD, involves hundreds of cell-type-specific enhancers that respond to neuronal activity. Once selected, enhancers are active throughout adulthood, suggesting long-lasting effects on gene regulation in the adult brain.
Taken together, these two studies implicate activity-dependent neuronal gene expression with neurological conditions. The Greenberg lab plans to follow up on their findings by mapping neurological disease-associated sequence variants within neuronal activity-dependent regulatory elements to further dissect the link between neuronal activity, gene regulation, and development — both in health and disease.
Jonathan Schneiderman is Associate Director of Research in the Neurobiology Department at Harvard Medical School.
Learn more in the original research articles:
Activity-dependent regulome of human GABAergic neurons reveals new patterns of gene regulation and neurological disease heritability.
Boulting GL, Durresi E, Ataman B, Sherman MA, Mei K, Harmin DA, Carter AC, Hochbaum DR, Granger AJ, Engreitz JM, Hrvatin S, Blanchard MR, Yang MG, Griffith EC, Greenberg ME. Nat Neurosci. 2021 Mar;24(3):437-448. doi: 10.1038/s41593-020-00786-1. Epub 2021 Feb 4.
An Activity-Mediated Transition in Transcription in Early Postnatal Neurons.
Stroud H, Yang MG, Tsitohay YN, Davis CP, Sherman MA, Hrvatin S, Ling E, Greenberg ME. Neuron. 2020 Sep 9;107(5):874-890.e8. doi: 10.1016/j.neuron.2020.06.008. Epub 2020 Jun 25.